Scientific Poster
>
Development and Implementation of the S-PLEX Platform for Converting Standard Immunoassays to High-Sensitivity Assays AAPS 2019
Development and Implementation of the S-PLEX Platform for Converting Standard Immunoassays to High-Sensitivity Assays
Download ↓
1. Purpose
Measurements of circulating biomarkers in biofluids are widely used as an essential aspect of pharmaceutical research and drug development applications. While many biomarkers are readily measurable in the pg/mL to µg/mL range using common immunoassay techniques, some analytes are present at much lower concentrations, making them difficult to detect. Recent advances have enabled measurements of proteins in the fg/mL range but the number of available assays is limited and they can be difficult to develop. We describe an electrochemiluminescence-based platform that enables the conversion of standard sandwich immunoassays to high-sensitivity assays using common reagents such as biotinylated antibodies and streptavidin-coated plates. The platform has been analytically validated over multiple lots of assay components and has been tested on a set of 50 standard immunoassays, with the majority of assays showing significant (10- to 1000-fold) enhancement in sensitivity.
 View More
View More
 View Less
View Less
2. Objectives
MSD’s next generation S-PLEX platform was developed with fg/mL sensitivity to enable measurement of low abundant analytes in normal donor samples that previously are not measurable by standard immunoassay methods.
3. Methods
MSD’s electrochemiluminescence detection technology uses SULFO-TAG labels that emit light upon electrochemical stimulation initiated at the electrode surfaces of microplates.
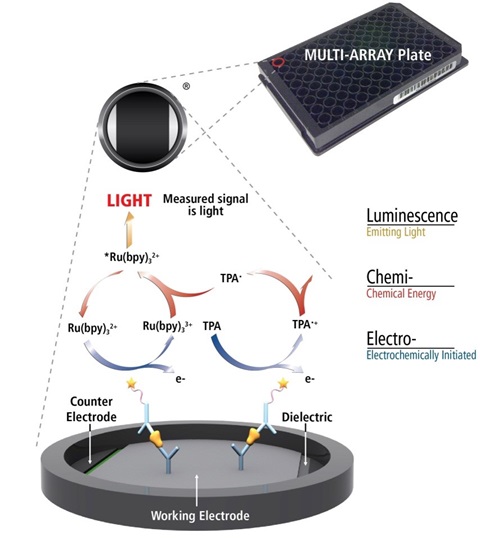
Electrochemiluminescence Technology
-
Minimal non-specific background and strong responses to analyte yield high signal-to-background ratios.
-
The stimulation mechanism (electricity) is decoupled from the response (light signal), minimizing matrix interference.
-
Only labels bound near the electrode surface are excited, enabling non-washed assays.
-
Labels are stable, non-radioactive, and directly conjugated to biological molecules.
-
Emission at ~620 nm eliminates problems with color quenching.
-
Multiple rounds of label excitation and emission enhance light levels and improve sensitivity.
-
Carbon electrode surface has 10X greater binding capacity than polystyrene wells.
-
Surface coatings can be customized.
S-PLEX assay components such as plates, diluents, blockers, labels, and other reagents were specifically designed to enable conversion of standard immunoassays to high sensitivity assays. Multiple lots of the components were produced and characterized, and the protocol for the new S-PLEX format was optimized for robustness.
Fifty MSD immunoassays were converted to the S-PLEX format and evaluated. A group of 19 of these assays were selected for further characterization including measuring the limit of detection (LOD), lower limit of quantitation (LLOQ), upper limit of quantitation (ULOQ), dilution linearity, spike recovery and measurement of analyte concentrations in multiple sample matrices including serum, EDTA/heparin/citrate plasma, CSF, and stimulated cell supernatants (up to 72 samples for each assay). Concordance of the new S-PLEX assays with standard immunoassay formats was measured to demonstrate similar quantitation.
4. Sensitivity Improvements Using S-PLEX
Of the 50 assays converted to the S-PLEX format, 36 demonstrated sensitivities in the fg/mL range, and greater than 50% showed 10- to 1000-fold improvement over standard immunoassay formats. Representative assays demonstrating greater than 100-fold improvement in sensitivity are shown in the table.
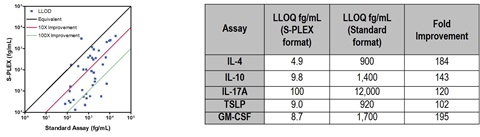
5. Assay Performance
Of the 50 assays converted to the S-PLEX format, 36 demonstrated sensitivities in the fg/mL range, and greater than 50% showed 10- to 1000-fold improvement over standard immunoassay formats. Representative assays demonstrating greater than 100-fold improvement in sensitivity are shown in the table.
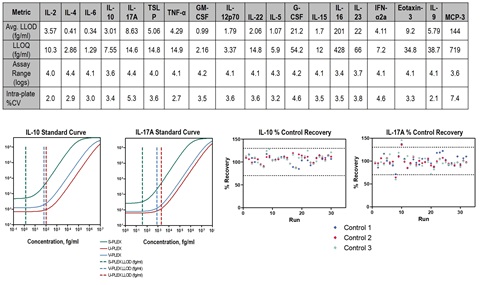
Top graphs compare representative standard curves for the IL-10 and IL-17A assays run in two standard immunoassay platforms (MSD U-PLEX and V-PLEX ) and run in S-PLEX. The S-PLEX assays demonstrated more that 20X improvement in sensitivity, with LODs of 1.4 fg/ml and 38 fg/ml for IL-10 and IL-17A respectively (compared to 67 to 2,070 fg/ml for the standard assays).
Assay reproducibility was assessed by running two replicates of high (QC 01), medium (QC 02) and low (QC 03) control samples per plate on nineteen plates spread over 6 months and four operators. The lower graphs show concentration % recovery of the control samples. 100% of the IL-10 and more than 90% of IL-17A assay controls recovered within 70%-130% of the expected values, demonstrating the high robustness of the S-PLEX platform.
6. Sample Testing
Normal serum and plasma samples were tested on the S-PLEX assays. Data from a number of proinflammatory cytokines is shown below. These cytokines typically have very low endogenous levels that may not be detectable with standard immunoassays. The enhanced S-PLEX sensitivity allows quantitation of normal levels in nearly all of the samples tested.
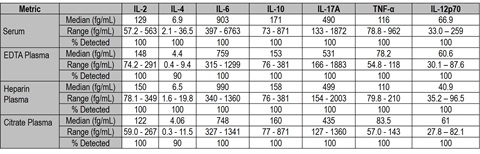
A comparison of normal sample levels of IL-10 (n=32) and IL-17A (n=16) in healthy donors measured on S-PLEX and two standard MSD immunoassay formats is shown below. Most samples with low endogenous levels were either undetectable or near the LOD of the standard assays, but readily detectable with the S-PLEX assays.

7. Sample Concordance
Sample concentrations measured on S-PLEX and standard immunoassay formats for IL-10 and IL-17A assays showed good correlation (r2 > 0.95, slopes 0.8-1.1). Representative concordance data from a single run of stimulated samples spiked into healthy donor sera are shown below.
8. Matrix Performance
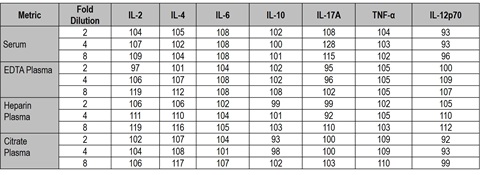
Dilution linearity was evaluated in serum and plasma from four donors and is shown below as average percent recovery in the matrices tested. Recovery ranged from 84% to 119% across all assays and dilutions tested.
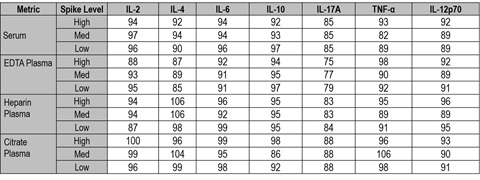
Spike recovery was also evaluated in different sample matrices for four donors. Average percent recovery is shown below, and ranged from 75% to 106%.
9. Conclusions
S-PLEX is a highly sensitive immunoassay format that can provide detection limits in the fg/ml range. Standard immunoassays can easily be converted to the S-PLEX format; improvements in sensitivity up to 1000-fold have been demonstrated.
The increased sensitivity of S-PLEX assays can allow quantitation of endogenous analytes which are not detectable using standard immunoassay formats.
The S-PLEX high-sensitivity format can be applied to bioanalytical assay formats other than sandwich immunoassays, with applications in various fields including pharmacodynamics, anti-drug antibody (ADA) and other anti-antibody detection assays.
Customer Service/Orders
Scientific/Technical Support
Instrument Support
Company Headquarters