Methods
Electrochemiluminescence Technology
MSD’s electrochemiluminescence detection technology uses SULFO-TAG™ labels that emit light upon electrochemical stimulation initiated at the electrode surfaces of MULTI-ARRAY® and MULTI-SPOT® microplates.
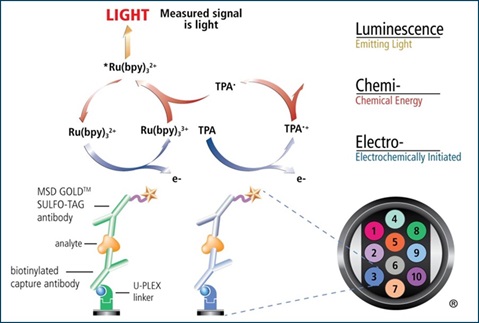
•Minimal background, combined with strong response to analyte, yields high signal-to-noise ratios.
•The stimulation mechanism (electricity) is decoupled from the response (light signal), minimizing matrix interference.
•Only labels bound near the electrode surface are excited, enabling non-washed assays.
•Labels are stable, non-radioactive, and directly conjugated to biological molecules.
•Emission at ~620 nm eliminates problems with color quenching.
•Multiple rounds of label excitation and emission enhance light levels and improve sensitivity.
U-PLEX® Immuno-Oncology Protocol
The U-PLEX assay platform uses 10 unique linkers that specifically bind to individual spots, enabling simple and flexible creation of multiplex immunoassays.
Couple and Coat the U-PLEX Plate:
- Add 200 µL of the biotinylated capture antibody to 300 µL of the assigned linker. Vortex. Incubate for 30 minutes.
- Add 200 µL of Stop Solution and vortex. Incubate for 30 minutes.
- Combine each U-PLEX-coupled antibody solution into a single tube and vortex. Add 50 µL of multiplex coating solution to each well.
- Incubate with shaking for 1 hour then wash the plate.
Complete the Assay:
- Add 50 µL of sample, calibrator, or control to each well.
- Incubate the plate for 2 hours, then wash the plate.
- Add 50 µL of detection antibody solution to each well.
- Incubate the plate for 1 hour, then wash the plate.
- Add 150 µL of MSD® Read Buffer to each well and read the plate.

Results
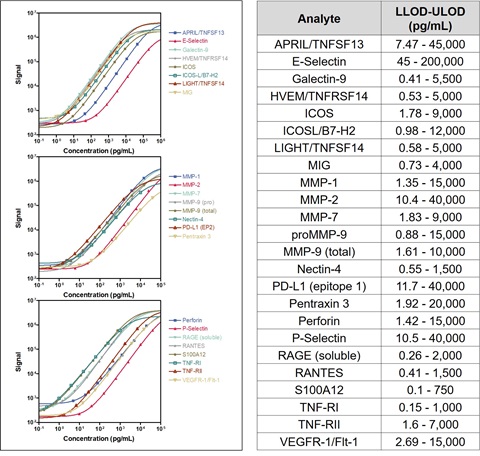
Assay Characteristics
Calibrator curves, lower limit of detection (LLOD), and upper limit of detection (ULOD) for 24 new human immuno-oncology assays are shown below. LLODs were calculated from 3 runs each with >20 blank wells. Control samples for each assay showed expected precision and accuracy, with intra-run CVs less than 10%, inter-run CVs less than 25%, and recoveries largely within 70-130% of target concentrations (data not shown).
U-PLEX Biomarker Compatibility
Compatibility with existing human U-PLEX immuno-oncology assays was tested using dynamic range, sensitivity, sample detection, and non-specific binding as performance criteria. As a result, the U-PLEX Immuno-Oncology Group 1 (human) panel was expanded to 131 assays (see the table below) that can be multiplexed together.

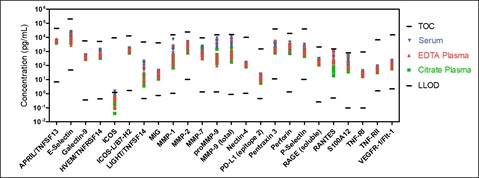
Native Sample Testing
Immuno-oncology assays were evaluated for the ability to detect their respective analytes in human serum, EDTA plasma, and citrate plasma samples. Sample concentrations (pg/mL) were plotted with the top of curve (TOC) and LLOD for each analyte. Samples were diluted 4-fold except for ICOSL/B7-H2, MMP-2, proMMP-9, MMP-9 (total), P-Selectin, RANTES, S100A12, TNF-RI and TNF-RII assays where samples were diluted 100-fold. ICOS was not detected in human serum and plasma samples. All other analytes were detected irrespective of the type of matrix.
Human tissue lysate samples (6.25 µg) derived from different normal tissues (blue symbols) and tumor tissues (red symbols) were tested. Sample concentrations (pg/mL) were plotted with TOC and LLOD values for each analyte. All analytes were detected in most of the tissue lysate samples.
Dilution Linearity
Serum and EDTA plasma samples were spiked with calibrator and diluted 2, 4, 8, and 16-fold before testing. Sample concentrations were normalized to the 4-fold sample dilution. For ICOS-L/B7-H2, MMP-2, proMMP-9, MMP-9 (total), P-Selectin, RANTES, S100A12, TNF-RI and TNF-RII, unspiked samples were diluted 50, 100, 200 and 400-fold. Sample concentrations for these were normalized to the 100-fold sample dilution. Most analytes recovered within 70-130% in each type of sample. Recovery of MIG improved with the addition of 0.1% Triton X-100 (data not shown).
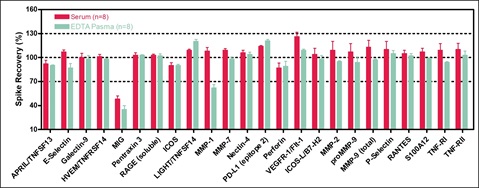
Spike Recovery
Normal human serum and EDTA plasma samples were spiked with calibrators at 3 levels (high, mid, and low). Spike recovery values for the three spike levels were averaged and plotted. Recovery of most analytes was within 70-130% in each sample type.
Assay Interference and Competition
Immuno-oncology assays were evaluated for interference and competition with therapeutic antibody drugs and homologous and/or related analytes. Assay interference was measured by comparing recovery of analyte in the presence of a wide concentration range of the potential interferent. Competition was measured by comparing human serum and plasma sample concentrations in singleplex and multiplex formats. Percent change from control was reported in the table below (% change >50% shaded red, <50% shaded yellow, <20% shaded green). Testing with therapeutic antibody drugs demonstrated that the PD1 (epitope 1) assay is more resistant to Nivolumab and Pembrolizumab than PD1 (epitope 2). Similarly, PD-L1 (epitope 2) is more resistant to Atezolizumab than PD-L1 (epitope 1).
Conclusions
The U-PLEX immuno-oncology assay portfolio has expanded to 51 assays with the addition of twenty-four new human assays. U-PLEX immuno-oncology assays can be used in singleplex and multiplex formats and can be run in combination with 80 additional biomarker assays bringing the total number of compatible assays to 131. These assays enable researchers and drug developers to simultaneously measure immuno-oncology analytes along with cytokines, chemokines, and inflammatory markers.