Scientific Poster
>
Performance Summary for Multiplexed, Isotype-Specific Research-Use Serology Assays for Detection of Autoimmune Reactivities FOCIS 2020
Performance Summary for Multiplexed, Isotype-Specific Research-Use Serology Assays for Detection of Autoimmune Reactivities
Download ↓
1. Abstract
Background: Autoimmune diseases affect over 50 million Americans. The presence of specific autoantibodies can predict disease onset in at-risk individuals (e.g., type 1 diabetes, systemic lupus erythematosus, and celiac disease) and assist in distinguishing disorders with similar clinical features (e.g., type 1 versus type 2 diabetes). Multiplexed serology panels were developed for profiling IgG, IgA, and IgM autoantibody responses against 24 different autoantigens associated with important autoimmune diseases or connective tissue disorders (72 assays in total). These research-use-only panels were developed on the sensitive MSD® MULTI-ARRAY technology platform and included measurements using bridging and/or classical serology assay formats. Serum-derived calibrators were used for quantitative measurement of each reactivity and as positive/negative controls for assay performance tracking. These panels were applied to three sample sets: (1) samples from a drug trial (T1DAL) for type 1 diabetes (ITN), (2) samples from a clinical study on gluten-free diets for celiac disease (Harvard University), and (3) matched lupus disease samples from individuals at or without flare (University of Minnesota).
Results: Assay performance data for calibrators, controls, and test samples are presented to demonstrate the reproducibility and robustness of the assay methods. Selected data are shown for markers that distinguish subgroups within a study set.
Conclusion: The multiplexed isotype-specific autoantibody assays provided reliable, quantitative, and sensitive measurement of 72 specific reactivities while requiring less than 200 μL of serum/plasma per sample. This platform provides a new tool that can be used in autoimmune disease research to broadly profile autoimmune reactivities in each sample.
 View More
View More
 View Less
View Less
2. Methods
MSD’s electrochemiluminescence (ECL) detection technology uses SULFO-TAG™ labels that emit light upon electrochemical stimulation initiated at the electrode surfaces of MULTI-ARRAY® and MULTI-SPOT® microplates.
Electrochemiluminescence Technology
- Minimal non-specific background and strong responses to analyte yield high signal-to-background ratios.
- The stimulation mechanism (electricity) is decoupled from the response (light signal), minimizing matrix interference.
- Only labels bound near the electrode surface are excited, enabling non-washed assays.
- Labels are stable, non-radioactive, and directly conjugated to biological molecules.
- Emission at ~620 nm eliminates problems with color quenching.
- Multiple rounds of label excitation and emission enhance light levels and improve sensitivity.
- Carbon electrode surface has 10X greater binding capacity than polystyrene wells.
- Surface coatings can be customized.
Serology Assay Formats
The panel formats, Bridging Simultaneous, Bridging Sequential, or Classical Serology, are illustrated above, all using MSD’s U-PLEX technology. We combine the performance advantages of the MSD serology assays with the sample-sparing advantages of MSD multiplexed assays. Simultaneous detection of multiple autoantigen reactivities minimizes the amount of sample needed (<25 µL of diluted sample to detect all reactivities per panel, in duplicate). Samples are tested, along with MSD human serum-derived positive and negative controls and calibrators, to quantitate the autoimmune responses for each analyte and to assess assay reproducibility.
3. Selected Assays
The samples were tested on multiple panels to measure reactivity to 24 autoantigens listed below, detecting IgA, IgG and IgM isotypes of each autoimmune reactivity, using the assay formats indicated. Smith and MPO reactivities were measured in both bridging and classical serology formats. Thirty-eight samples were tested in duplicate on each assay plate along with an MSD calibrator and MSD positive and negative control samples. Samples for the bridging simultaneous assays were acid treated. Sample dilutions used ranged from 6- to 30-fold.
4. Calibrators and Controls
Calibrators and controls were prepared from screened human serum/plasma samples. Multiple individual samples were sourced and tested for reactivity to each antigen to identify ones with high levels of autoantibodies. In most cases, each isotype (IgA, IgG, or IgM) required unique samples. The sample signals had to be high enough for sufficient dynamic range to remain for calibrator materials following pooling of samples for the individual reactants in a panel of assays. No recombinant material was available for use as a calibrator or control.
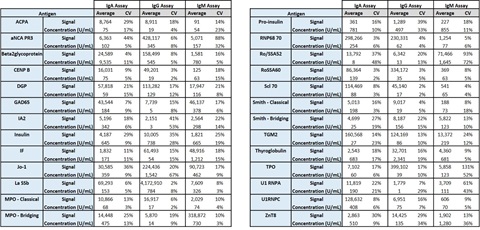
5. Positive Control Performance Summary
Positive and negative controls were run in duplicate per plate, over 6 plates per panel, run across 6 days, by 3 analysts. The summarized positive control signals and calculated concentrations demonstrate robust performance for most assays.
6. Sample Cohorts
T1DAL/ITN045AI Cohort - INDUCING REMISSION IN NEW ONSET T1DM WITH ALEFACEPT (Amevive) [Benaroya Research Institute at Virginia Mason (BRI) and ITN]
This was a multi-center, prospective, double-blind, placebo-controlled, 50-patient, 2:1 randomized, phase II clinical trial for individuals with recent-onset Type 1 Diabetes, aged 12−35 years. Participants received weekly injections of alefacept (15 mg) or placebo for 12 weeks, followed by a 12-week pause before resuming another 12 weeks of dosing, for a total course of 24 weeks of alefacept or placebo. (Rigby et al., 2013) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3957186/pdf/nihms543103.pdf). Alefacept is a dimeric fusion protein consisting of the leukocyte function antigen-3 protein and Fc portion of human IgG1 that blocks the T-cell CD2 receptor, thus preventing T-cell proliferation. It also induces apoptosis of effector memory T cells.
MSD Project: To measure autoantibodies during Alefacept treatment and stratify with response (change in disease progression) to see if there is any correlation. Samples were provided blinded with respect to treatment groups and outcomes.
Celiac Disease Cohort (Beth Israel Deaconess Medical Center)
Newly diagnosed, untreated celiac disease. (20 samples)
Patients were confirmed positive by anti-TGM2 (TTG) IgA serology and small bowel biopsy demonstrating characteristic changes of celiac disease histopathology with villus atrophy (Marsh III lesion). A gluten-free diet (GFD) had not yet been initiated or was initiated no more than 4 weeks prior to serum collection.
Treated celiac disease. (20 samples)
These are patients with known celiac disease at a follow-up visit with clinically indicated celiac serology request/sample collection for monitoring serologic response to the GFD. The GFD had been initiated at least 12 months prior to serum collection.
Non-celiac (NC) controls. (20 samples)
Celiac disease in these patients was excluded clinically and by negative celiac serology, gastro-intestinal symptoms caused by confirmed gastrointestinal disorders, and ascertaining they were ingesting a normal, gluten-containing diet prior to serum collection.
MSD Project: To identify markers of celiac disease and markers of GFD treatment response.
Systemic Lupus Erythematosus (SLE) Cohort (University of Minnesota)
Samples included sera from 15 SLE patients. For each patient, samples were from one flare time point (HIGH) and one non-flare time point (LOW). The visit number at which samples were collected and their SLEDAI scores were provided. The HIGH sample may have been collected from a time point that followed or preceded the LOW sample in any given patient. Additionally, 15 control samples from matched normal subjects were provided (single time point for each).
MSD Project: To evaluate the performance of SLE-related markers in the classical serology and/or bridging formats, identify potential novel SLE markers, and identify potential markers of SLE flare development.
7. T1DAL Testing – Sample Reproducibility
All samples were tested in duplicate on all assays. Replicate measurements were highly reproducible along the dynamic ranges of each assay. A representative set of data from testing of the T1DAL samples is shown below for three type 1 diabetes and one celiac disease markers. Median and mean estimated limits of detection across the multiple plates and runs for each assay are shown below each set of graphs.
8. Celiac Disease Study
Data are shown below for two classical celiac disease markers. Anti-TGM2 and anti-DGP antibody levels are highest in Untreated patient samples and clearly reduced in Treated patient samples. Levels in celiac patient samples were elevated relative to non-celiac (NC) controls regardless of treatment status except for IgM reactivities that were comparable for NC and treated patient samples.
9. SLE Study
The classical SLE markers were shown to separate SLE from matched control samples very efficiently. Data for the top three performing markers are shown. This is likely the first demonstration of the use of a bridging serology assay for an SLE marker (Smith IgG).

When markers were ranked by the Mann-Whitney-Wilcoxon test for their ability to distinguish SLE flare from non-flare samples, the top predictors were not the classical SLE markers, but were IAA-IgM and MPO-IgA, autoantigens associated with type 1 diabetes and vasculitis, respectively. Each line below represents one patient, connecting the flare and non-flare marker concentrations.
10. Conclusion
Based on experience with the sample testing discussed above, all the listed markers (24 autoantigen reactivities x 3 isotypes) can be tested in samples in duplicate using a total of ~150 µL serum/plasma, yielding quantitative measures for each reactivity. These results demonstrate the sample-sparing capability of the approach described as well as the robustness, reproducibility, and versatility of the MSD detection platform. The broad applicability of the bridging serology approach with its inherent advantages, including increased specificity, is also demonstrated for markers that are not normally assessed in this format.
The research reported herein was supported by the NIAID of the NIH under Award #1 U24 AI 118660. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Customer Service/Orders
Scientific/Technical Support
Instrument Support
Company Headquarters