Scientific Poster
>
Highly Sensitive Assay for Neurofilament Light in Serum and Plasma TBI 2021
Highly Sensitive Assay for Neurofilament Light in Serum and Plasma
Download ↓
1. Abstract
Neurofilament light protein (NF-L) is found at elevated levels in CSF and blood in various neurologic conditions, such as traumatic brain injury (TBI), neurodegeneration, and hypoxia. Assays for NF-L are useful research tools and may be developed as clinical tests. CSF collection requires invasive lumbar punctures, therefore assays capable of measuring NF-L in blood are preferred. This requires highly sensitive assays because NF-L concentrations in blood are more than two orders of magnitude lower than in CSF.
MSD has developed and characterized a highly sensitive and specific immunoassay for measuring NF-L in CSF, serum, and plasma based on MULTI-ARRAY® technology. This easy-to-use assay is performed in 96-well plates using standard liquid-handling techniques and has a simple 4-step workflow with a total of 3 hours incubation time. Owing to single-digit pg/mL sensitivity, this assay can detect NF-L in human plasma and serum samples. The limit of detection (LOD) for the assay is 2.8 pg/mL, determined by calculating the concentration corresponding to the average zero calibrator signal (N=20) plus 2.5 standard deviations. The lower limit of quantitation (LLOQ) is 7.5 pg/mL and is defined as the lowest concentration with a total error (%CV + |100-%Recovery|) of 25% or less. The median %CV for 283 plasma samples from neurocritical patients tested in duplicate was 2.7%. The specificity of the assay was confirmed by demonstrating signal reduction after depleting samples with two different anti-NF-L antibodies not used in the assay. The assay was further characterized by reproducibility, spike recovery, and dilution linearity testing. The NF-L assay correlated well with an assay from Quanterix for a set of plasma samples obtained from TBI patients (R2 = 1.00).
This easy-to-use NF-L assay enables measurement of low NF-L levels in serum and plasma samples and will prove useful for studying neuron injury or neurodegeneration.
 View More
View More
 View Less
View Less
2. Methods
MSD’s electrochemiluminescence detection technology uses SULFO-TAG labels that emit light upon electrochemical stimulation initiated at the electrode surfaces of MULTI-ARRAY and MULTI-SPOT microplates.
Electrochemiluminescence Technology
-
Minimal non-specific background and strong responses to analyte yield high signal-to-background ratios.
-
The stimulation mechanism (electricity) is decoupled from the response (light signal), minimizing matrix interference.
-
Only labels bound near the electrode surface are excited, enabling non-washed assays.
-
Labels are stable, non-radioactive, and directly conjugated to biological molecules.
-
Emission at ~620 nm eliminates problems with color quenching.
-
Multiple rounds of label excitation and emission enhance light levels and improve sensitivity.
-
Carbon electrode surface has 10X greater binding capacity than polystyrene wells.
-
Surface coatings can be customized.
3. Calibration Curve
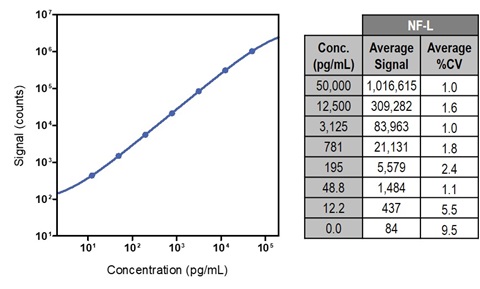
Calibrator curve signals and %CV values are an average of 7 runs. The %CV values represent average intraplate %CV for two replicates.
Protocol
- Coat plate with capture antibody.
- Add calibrators and samples (50 µL per well). Incubate 2 hours at room temperature (RT).
- Wash and add detection antibody solution (50 µL per well). Incubate 1 hour at RT.
- Wash and add read buffer (150 µL per well). Analyze with MSD instrument.
4. Assay Range
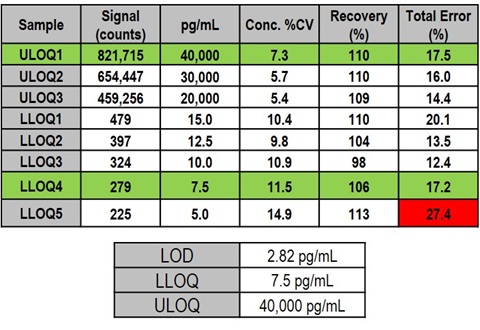
Samples used to determine limits of quantitation (LOQ) were prepared by making dilutions of NF-L calibrator in assay diluent.
Data are from 9 runs with duplicate wells (18 measurements) performed by three operators. LLOQ was defined as the lowest concentration with a Total Error (%CV + |100-%Recovery|) of 25% or less. ULOQ was defined as the highest concentration with a Total Error of 25% or less. Percent recoveries of LOQ samples (100 x measured concentration/assigned concentration) were within 80-120%.
5. Reproducibility
ECL signals for calibrators varied by less than ±20% over eight plates and four days. In testing of 283 plasma samples from neurocritical patients, median %CV for duplicates was 2.7%.
6. Specificity
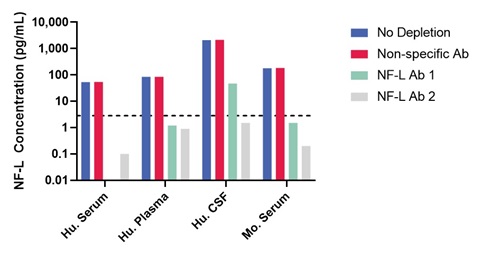
Depletion experiments were performed using two different antibodies known to be specific for NF-L and which were not the same as the antibodies used in the MSD NF-L assay.
Depletion was performed using human serum, plasma, and CSF from apparently healthy donors. Samples were depleted using antibody-labeled magnetic beads. Measured levels of NF-L were similar for the undepleted sample and samples depleted with the control antibody that does not bind NF-L (non-specific antibody). All of the donor samples were measurable and above the LLOQ prior to depletion. Both NF-L specific antibodies completely depleted the serum and plasma samples to below the LOD of the assay (dashed line). These data indicate that the MSD NF-L assay is highly specific for the NF-L protein.
7. Dilution Linearity and Parallelism
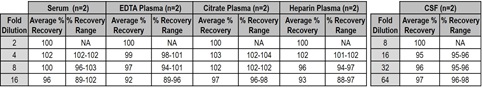
For dilution linearity testing, serum and plasma samples from apparently healthy individuals were spiked with NF-L calibrator and then diluted 2-, 4-, 8-, and 16-fold with assay diluent. Percent recovery at each dilution was normalized to the 2-fold diluted concentration. For parallelism, unspiked CSF samples from apparently healthy individuals were diluted 8-, 16-, 32-, and 64-fold with assay diluent. Percent recovery at each dilution was normalized to the 8-fold diluted concentration. Average recovery of diluted samples ranged between 89-104% across all samples. The results indicate that the assay can accurately measure NF-L in biofluids with little or no matrix effect.
% Recovery = (measured concentration / expected concentration) x 100. NA = not applicable.
8. Spike Recovery
Serum and plasma samples from apparently healthy individuals were spiked with NF-L calibrator at three levels. Average recovery of spiked samples was 89-140% for serum, 96-143% for EDTA plasma, 96-124% for citrate plasma and 70-112% for heparin plasma. The results indicate that the assay can accurately measure NF-L in serum and plasma samples with little or no matrix effect.
% Recovery = (measured concentration / expected concentration ) x 100.
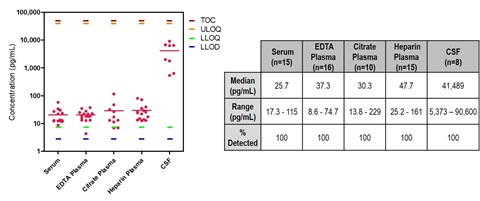
9. NF-L Concentrations in Serum and Plasma Samples
Normal serum and plasma samples were tested at two-fold dilution. Cerebral spinal fluid (CSF) was tested at 10-fold dilution. NF-L was detected in all normal samples tested.
TOC = top of calibration curve.
10. Correlation Across Platforms
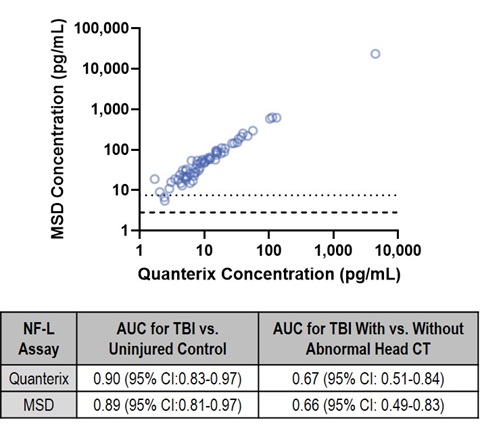
A blinded comparison of the MSD and Quanterix assays for NF-L was performed on 66 plasma samples obtained from uninjured controls (n=23) and patients who presented to a trauma center with traumatic brain injury (TBI) (n=43). Samples had been analyzed independently using the Simoa Human Neurology 4-Plex A assay manufactured by Quanterix (LOD = 0.038 pg/mL; LLOQ = 0.175 pg/mL). Samples were analyzed at MSD using the NF-L electrochemiluminescence-based immunoassay without knowledge of the prior measurements. Measurements of NF-L in plasma made using the MSD and Quanterix platforms were highly correlated (Pearson correlation = 1.00). Both NF-L assays differentiated between TBI patients and normal controls as well as TBI patients with and without abnormal head CT scans. While the two methods were strongly correlated, the MSD assay generated concentration values that were roughly three times higher than the Quanterix assay.
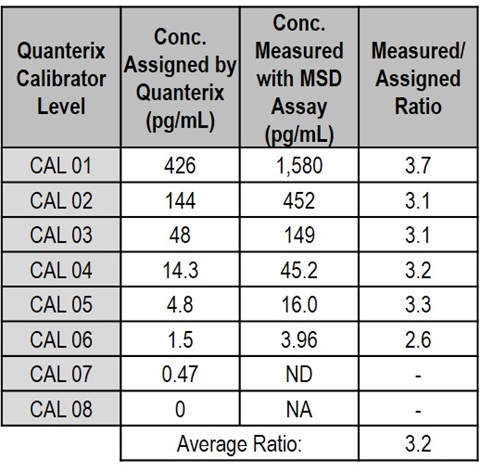
To investigate differences in quantitation between the assay platforms, Quanterix calibrator standards were measured with the MSD NF-L assay. Concentrations of the Quanterix calibrator standards measured with the MSD calibration curve were 3.2 fold higher than their assigned values. This result shows that the differences in measured concentrations are due to differences in the assignment of calibrator concentration for the Quanterix and MSD calibrators. Due to this difference in calibration, the MSD assay measured levels of NF-L in normal human plasma (n=8) as 14.1±1.1 pg/mL using the Quanterix calibrators and 42.7±3.5 pg/mL using the MSD calibrators.
11. Conclusions
Using MULTI-ARRAY technology, MSD has developed and characterized a highly sensitive, easy-to-use immunoassay for quantitating NF-L in serum, plasma, and CSF.
The assay achieves sufficient sensitivity to detect NF-L in normal serum and plasma on a robust platform.
The assay should prove broadly useful for studying neuronal injury and neurodegeneration.
Customer Service/Orders
Scientific/Technical Support
Instrument Support
Company Headquarters