Scientific Poster
>
Development and Validation of Multiplexed Metabolic Assay Panel Using ECL Technology AAPS 2020
Development and Validation of Multiplexed Metabolic Assay Panel Using ECL Technology
Download ↓
PURPOSE
The increased prevalence of metabolic-related diseases including diabetes, obesity, and metabolic syndrome has driven demand for the sensitive measurement of biomarkers linked to these disease states. The expression levels of these biomarkers, produced in the gut, adipose tissue, and brain, are frequently altered during disease and can be measured in plasma and serum. To address the need for sensitive and validated biomarker assays, we developed individual and multiplexed assays targeting seven metabolic biomarkers for use in human, nonhuman primate, canine, and rodent samples. The metabolic panel consists of assays for Insulin, C-peptide, GLP-1 Active (7-36 amide), GIP Active (1-42), Glucagon, PP, and Leptin. Using this panel, endogenous analyte levels can be measured and monitored throughout the metabolic process with as little as 25 μL of sample.
 View More
View More
 View Less
View Less
METHODS
MSD’s electrochemiluminescence detection technology uses SULFO-TAG™ labels that emit light upon electrochemical stimulation initiated at the electrode surfaces of MULTI-ARRAY® and MULTI-SPOT® microplates.

Electrochemiluminescence Technology
-
Minimal non-specific background and strong responses to analyte yield high signal-to-background ratios.
-
The stimulation mechanism (electricity) is decoupled from the response (light signal), minimizing matrix interference.
-
Only labels bound near the electrode surface are excited, enabling non-washed assays.
-
Labels are stable, non-radioactive, and directly conjugated to biological molecules.
-
Emission at ~620 nm eliminates problems with color quenching.
-
Multiple rounds of label excitation and emission enhance light levels and improve sensitivity.
-
Carbon electrode surface has 10X greater binding capacity than polystyrene wells.
-
Surface coatings can be customized.
Challenges associated with multiplexing were solved through epitope mapping, extensive antibody screening followed by antibody engineering to enhance sensitivity anAverage intra-run %CV is the average %CV of the control replicates within an individual run across 48 runs (three kit lots).
Inter-run %CV is the variability of controls across 12 runs within a single kit lot (across three kit lots).
Inter-lot %CV is the variability of controls across 3 kit lots (total of 48 runs).
d specificity, and substantial diluent optimization to reduce matrix effects. Calibrators were anchored to in-house or industry-accepted reference standards. Analytical validation was performed across multiple lots and included testing of dynamic range, matrix tolerance, reproducibility, and limits of quantitation (LOQ). Specificity was tested against common metabolic markers and their structurally related analogs and fragments. Assay robustness was confirmed by varying temperatures and incubation times, and testing of other common use-case scenarios including the presence of interfering agents.
RESULTS
We developed assays for seven metabolic markers that are highly specific and demonstrate little interference from other blood components. These assays have dynamic ranges of over three logs and best-in-class sensitivities with lower limits of quantitation (LLOQ) for key metabolic assays such as Insulin, Glucagon, and GLP-1 Active (7-36 amide) (0.07 µIU/mL, 0.33 pM, and 0.10 pM, respectively). These highly sensitive assays permit accurate quantitation even in populations with suppressed secretion such as fasting, diabetic, or heart disease groups. Accuracy and precision were confirmed by testing three levels of controls across multiple lots, and all controls had concentration intra-run and inter-run %CVs of less than 7% with 80-120% recovery of target concentrations. Matrix compatibility was verified through parallelism, dilution linearity, and spike recovery studies in serum or plasma treated with protease inhibitors. Owing to high cross-species homology for several analytes on the panel, matrix studies included multiple species. These studies demonstrated matrix tolerance and accurate quantitation for most of the seven assays (typically between 80-120%). Most of the seven metabolic assays exhibited minimal cross-reactivity and interference from analytes that share significant sequence homology.
Calibration Curves and Limits of Detection
The figure to the right demonstrates typical calibration curves for the analytes in the V-PLEX© Metabolic Kits. Data in the table include upper limit of quantitation (ULOQ), lower limit of quantitation (LLOQ), and ranges for the lower limits of detection (LLOD, n=3 runs each from a different kit lot). Calibrators were reconstituted and diluted serially (4-fold) to generate a 7-point standard curve for each panel.

The LLOD is a calculated concentration corresponding to the average signal 2.5 standard deviations above the background (zero calibrator). The ULOQ and LLOQ are established for each lot by measuring multiple levels near the expected LLOQ and ULOQ levels. The final LLOQ and ULOQ specifications for the product are established after assessment of all validation lots.
Specificity
To assess the specificity of each assay, the V-PLEX Metabolic Kit was tested for nonspecific binding to the following proglucagon metabolites and other general metabolic targets at 1,000 pg/mL. Cross-reactivity below the assay limit of quantitation is reported as less than LLOQ (< LLOQ).
In addition, cross-reactivity to the following analytes was below the LLOQ and are not included in the table below: mouse leptin, rat leptin, canine leptin, PYY (1-36), PYY (3-36), PYY (3-34), NPY, Resistin, IL-6, Leptin receptor, ApoJ, A2M, G-CSF, LIF, Oncostatin M, CNTF, IL-11, IL-12, Ghrelin (active), and Ghrelin (inactive).
 *GLP-1 (active) concentrations may be suppressed in the presence of Liraglutide concentrations that are higher than 50 pM. ‡Cross-reactivity of Glucagon and circulating glicentin (1-61) is expected due to sequence similarities. †Major Proglucagon Fragment
*GLP-1 (active) concentrations may be suppressed in the presence of Liraglutide concentrations that are higher than 50 pM. ‡Cross-reactivity of Glucagon and circulating glicentin (1-61) is expected due to sequence similarities. †Major Proglucagon Fragment
Sample Testing
To assess the performance of the V-PLEX assays, human samples of P800-collected EDTA plasma from apparently healthy individuals obtained from a commercial source were diluted 2-fold and tested on the V‑PLEX Metabolic Kit.
Plasma samples were collected from different individuals at different fasting time points (2.0 – 14.5 hours). The sample concentration prior to dilution factor adjustment is displayed; see Figure 2, below. The results are in agreement with the expected concentrations reported in the literature and the majority of samples contained analyte levels above the LLOQ of each assay.
Matrix Performance
PARALLELISM, DILUTION LINEARITY, AND SPIKE RECOVERY
Serum and plasma samples collected in P800 tubes from apparently healthy human donors were obtained from a commercial source. Owing to the sequence homology of multiple analytes in the panel across mammalian species, several common animal models were also assayed. Results from mouse, non-human primate (NHP), rat, and canine samples are similar to that on human samples (data not shown).
To assess parallelism, samples with high endogenous levels of analytes were diluted 2-fold, 4-fold, 8-fold, and 16-fold, before testing. Percent recovery at each dilution level was normalized to the 2-fold dilution-adjusted concentration.
To assess linearity , samples were spiked with calibrator and diluted 2-fold, 4-fold, 8-fold, and 16-fold, before testing. Percent recovery at each dilution level was normalized to the 2-fold dilution-adjusted concentration. The average percent recovery is based on samples within the quantitative range of the assay (below).

To assess recovery, samples were spiked with calibrator at three levels (high, mid, and low) then diluted 2-fold. The average % recovery for each sample type is reported along with %CV and % recovery range.
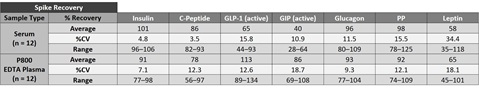
Accuracy and Precision
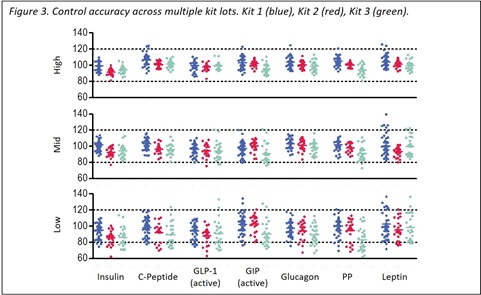
Quality control samples were prepared by spiking calibrator into a serum-free matrix at three levels (high, mid, and low) within the quantitative linear range of the assay. The controls were measured using a minimum of three replicates tested over multiple days and multiple operators for a total of at least 48 runs. The accuracy of control determinations fell within 20% of the expected concentration with precision of less than 20% CV in the majority of runs (Figure 3, below).
Accuracy is defined as the average measured control concentration for a given lot divided by the expected control concentration. The accuracy shown is an average of three replicates on a single plate. Runs were conducted using three kit lots by at least four different operators.
- Average intra-run %CV is the average %CV of the control replicates within an individual run across 48 runs (three kit lots).
- Inter-run %CV is the variability of controls across 12 runs within a single kit lot (across three kit lots).
- Inter-lot %CV is the variability of controls across 3 kit lots (total of 48 runs).

Conclusions
The V-PLEX Metabolic Panel 1 assays are highly specific, sensitive, and validated for use with serum and plasma from multiple species. Multi-lot analytical validation demonstrated consistent assay performance and accurate measurements of biomarkers associated with diabetes, obesity, and metabolic syndrome, making these assays valuable tools for basic research and preclinical studies. These metabolic assays include Insulin, GLP-1 Active (7-36 amide), and Glucagon assays with best-in-class sensitivity and specificity.
Customer Service/Orders
Scientific/Technical Support
Instrument Support
Company Headquarters