2. Methods
Co-culture mode set-up, optimization, and contrived sample testing
For co-culture model set-up, effector cells (TALL-104, NK-92, Jurkat) and target cells (MCF-7, PC3, U87MG, K562, HEK293) were cultured at various densities and effector-to-target (E:T) ratios in serum-depleted basal media conditions for 24 hours. The cell culture supernatant was collected to assess cellular viability using an ATP-based assay with glow-type luminescent signal (CellTiter-Glo, PromegaCorp). The assay was optimized from conditions established using the CellTiter-Glo assay for density and E:T ratio.
Three E:T ratios were selected for testing and functional assay characterization using harvested cell culture lysates and supernatants using CellTiter-Gloas well as Meso Scale Discovery’s assays for the following targets:

Electrochemiluminescence (ECL) Technology
- High sensitivity: Multiple rounds of label excitation and emission enhance light levels and improve sensitivity.
- Broad dynamic range: High and low abundance analytes can be measured without multiple sample dilutions.
- Low background: Electronic stimulation of detection label is decoupled from the output signal (light) resulting in extremely low background.
Samples were assayed in biological triplicate. For cell lysate samples, the triplicates were pooled and assayed at 2.5 μg protein/well and assayed in technical duplicate. For cell culture supernatants, collected supernatant was diluted 10 to 40-fold in assay diluent and 25 μL of sample was added to the assay plate.
Multiplex U-PLEX CAR-T Combos (Multiplex Assays)
Biotinylated capture antibodies are each coupled to U-PLEX Linkers, which self-assemble onto unique array elements (or “spots”) on the U-PLEX plate. Leveraging U-PLEX’s multiplexing platform conserves valuable samples by allowing up to ten determinations per well.

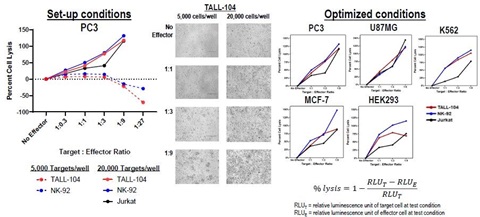
3. Co-culture set-up and optimization
The co-culture assays were optimized at multiple cell densities and E:T ratios using the CellTiter-Gloassay and visual inspection. We found the operating range of the co-culture assay to be optimal when seeding 20,000 target cells per well and between a 0.3-9:1 E:T ratio for the assay. All effector cell types tested impacted viability of the target cell in the co-culture model in a dose-dependent manner at the highest E:T ratio ranging between 69%-148% percent cell lysis after 24 hours co-culture.
4. Apoptotic index, KI-67, and CellTiter-Glo correlation
Whole cell lysate was collected from co-cultured conditions using Trypsin-EDTA for adherent cell lines and centrifugation. For suspension cells, co-cultures were centrifuged. Ice cold lysis buffer was added to cell lysates to extract intracellular protein. 2.5 μg protein per well from test conditions was assayed for cleaved and total caspase 3 and for KI-67. Dose-dependent responses in apoptotic cell death, expressed as the apoptotic index (ratio of cleaved to total caspase 3), were observed for TALL-104 for Jurkat cells. Proliferation of effector cells, measured by KI-67 levels, also increased in a dose-dependent fashion indicating that target engagement activated TALL-104 and NK-92 cell proliferation. Some correlation was observed between the CellTiter-Glo results and MSD’s apoptosis assay suggesting that it is critical to monitor cell death in cellular immunotherapy assays. Surprisingly, CellTiter-Glo predicted cell death of numerous target cell types co-cultured with Jurkatcells whereas the Meso Scale assays did not, as demonstrated by an R2value < 0.05.

5. Functional assessment of translationally-relevant biomarkers: CAR-T Combos

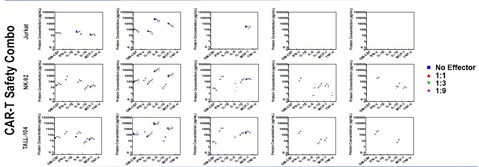
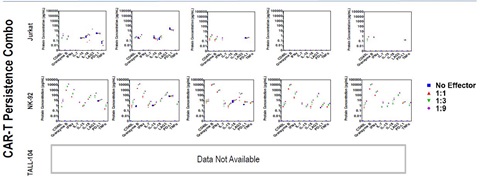
Supernatant was collected from co-cultures and assayed using U-PLEX CAR T Efficacy, Safety, and Persistence Combos. Biomarkers elevated in co-cultures with Jurkatcells were target-cell line dependent with no dose-dependent changes observed. Co-cultures of Jurkatwith HEK293 and MCF-7 had no impact on Efficacy, Safety, or Persistence cytokines or chemokines measured (below LLOD).
Target cell effects as well as dose-dependent effects were observed for TALL-104 and NK-92 cell types on biomarkers associated with Efficacy, Safety, and Persistence. With respect to efficacy, NK-92 cells induced secretion of at least 5 biomarkers (e.g., IFNγ, Granzyme B, Perforin, IL-12p70, and IP-10) in multiple co-culture models whereas TALL-104 commonly activated only 3 biomarkers (Granzyme B, IL-12p70 and Perforin) indicating different modes of action between NK-92 and TALL-104.
Similar observations are noted for CAR-T Safety where biomarkers associated with safety in co-cultures with Jurkatcells were target cell dependent. Interestingly, co-cultures of NK-92 and TALL-104 with HEK293 (assumed healthy) induced expression of IFNγ, IL-10, and MCP-1 indicating an inflammatory response which was unexpected for NK-92, but verifies the efficacy data. These results for TALL-104 and NK-92 were also dose-dependent.
Persistence of effector-cell function is demonstrated by significant increases in CD40L, IL-7, IL-15, and IL-18 when NK-92 are cultured with target cells. These data indicate that the cells are active and producing effector functions related to persistence and proliferation, confirming the KI-67 result.
7. Conclusions
- Cellular immunotherapy co-culture models are robust, reproducible and require multiple assays to down-select potential engineered candidates.
- The ratio of cleaved to total caspase3 (apoptotic index) maybe superior to other cell death assays in assessing a cellular immunotherapy’s ability to kill target cells in co-culture settings.
- Dose-dependent effects of a cellular immunotherapy can be rapidly assessed using MSD’s CAR-T Combos from in vitro screens related to efficacy, safety, and persistence.